Bloodstream Infections Caused by Stenotrophomonas Maltophilia a Seven-year Review
- Research article
- Open up Access
- Published:
Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations
BMC Infectious Diseases volume 15, Article number:69 (2015) Cite this article
Abstruse
Background
Stenotrophomonas maltophilia causes serious infections in immunocompromised hosts. Here, nosotros analyzed the clinical characteristics of S. maltophilia bloodstream infection (BSI) in patients with hematologic malignancies and evaluated in vitro synergistic effects of antimicrobial combinations.
Methods
We retrospectively reviewed all consecutive episodes of S. maltophilia BSIs in developed hematologic patients from June 2009 to May 2014, with in vitro susceptibility and synergy tests using high-throughput bioluminescence assay performed for available clinical isolates.
Results
Among 11,004 admissions during 5-yr period, 31 cases were identified as Due south. maltophilia BSIs. The incidence rate of Due south. maltophilia BSI was 0.134 cases/1,000 patient-days. Overall and attributable mortality of Due south. maltophilia BSI was 64.5% and 38.7%, respectively. Severe neutropenia (adjusted take chances ratio [HR] 5.24, p =0.013), shock at the onset of BSI (adjusted HR 6.05, p <0.001), and pneumonia (adjusted HR 3.15, p =0.017) were independent risk factors for mortality. In vitro susceptibilities to ceftazidime, levofloxacin, ticarcillin-clavulanic acrid (TIM) and trimethoprim-sulfamethoxazole (SXT) were 11.one%, 44.0%, forty.7%, and 88.nine%, respectively. MIC50/MIC90 for moxifloxacin and tigecycline were 1/four mg/L and 4/8 mg/L. The fifty% and 90% fractional inhibitory concentrations (FICl/FICninety) of clinical isolates confronting a combination of SXT and TIM were 0.500/0.750. For SXT plus levofloxacin or moxifloxacin, FIC50/FIC90 were 0.625/one.000 and 0.625/0.625, respectively.
Conclusion
S. maltophilia BSIs show loftier mortality, which is related to severe neutropenia, shock, and S. maltophilia pneumonia. Based upon drug susceptibility testing, the chief treatment of pick for S. maltophilia BSIs should be SXT in hematologic patients, rather than quinolones, with combination therapies including SXT serving as a feasible treatment option.
Background
Stenotrophomonas maltophilia is an emerging nosocomial pathogen in immunocompromised patients [ane-3]. Although Due south. maltophilia exhibits a limited pathogenicity in immunocompetent hosts, information technology has been shown to cause fatal infections in patients with hematologic malignancies. The overall mortality of S. maltophilia bloodstream infections (BSIs) ranges from 21 to 50%, with the bloodshed associated with neutropenia [4-6]. Failure to administration of early on susceptible antibiotics for S. maltophilia BSI can have clinical implications, equally Southward. maltophilia is naturally resistant to many antimicrobial agents including carbapenem.
Trimethoprim-sulfamethoxazole (SXT) is the antimicrobial agent of option for the treatment of South. maltophilia infections [7-nine]. Levofloxacin is too a viable handling option in cases where drug susceptibilities are known [10]. However, SXT is known to cause agin events related to os marrow suppression, which might delay recovery from neutropenia in patients with hematologic malignancies. Fluoroquinolone is ordinarily used as prophylaxis during stem prison cell transplantation (SCT) or chemotherapy. Every bit recent guidelines and experts have suggested that there are concerns about potential resistance to fluoroquinolone-based prophylaxis, this safe strategy tin pb to a limited effectiveness of levofloxacin in S. maltophilia infections [seven,11-13]. Data regarding the clinical characteristics and the treatment outcomes of S. maltophilia BSIs in hematologic patients who received quinolone prophylaxis remain insufficient.
As Southward. maltophilia BSIs are associated with a loftier mortality rate, and increased resistance to monotherapy, many groups accept suggested the need for combination antimicrobial therapies [7,fourteen,15]. Nonetheless, the effectiveness of combination therapy for Due south. maltophilia has not yet been established. Here, we investigated the clinical characteristics and outcomes related to Southward. maltophilia BSIs in patients with hematologic malignancies. Clinical isolates from these patients were so evaluated for in vitro susceptibilities with synergistic furnishings of several antimicrobial combinations to place potential therapeutic regimens that may better clinical outcomes.
Methods
Written report blueprint and hospital setting
We retrospectively reviewed medical records of all sequent episodes of Due south. maltophilia BSIs in adult patients with hematologic malignancies from June 2009 to May 2014 at the Catholic Blood and Marrow Transplantation Centre of Seoul St. Mary's Hospital.
Clinical data collection
Eligible patients included those with hematologic malignancies older than 19 years of age, with documented blood cultures positive for S. maltophilia. Clinical data obtained for each patient included historic period, sex, underlying diseases, severity and duration of neutropenia, length of hospital stay, simplified acute physiology score II (SAPS Two) at the onset of BSI, the presence of key venous catheters, organisms isolated from blood and the antimicrobial susceptibility, administered antibiotics, and survival status at thirty days afterwards the onset of BSI. The Institutional Review Board of Seoul St. Mary'due south Hospital approved the research protocol and waived the requirement for informed consent (KC13SISI0163).
S. maltophilia 16S rRNA gene analysis & pulsed-field gel electrophoresis
Bachelor clinical isolates underwent phylogenetic grouping determination and pulsed-field gel electrophoresis (PFGE). Clinical isolates were screened using a specific 16S rRNA cistron polymerase chain reaction (PCR) analysis, and sequenced to confirm taxonomic identities. PCR was performed using primers SM1f (five′-GTTGGGAAAGAAATCCAGC-three′) and SM4 (five′-TTAAGCTTGCCACGAACAG-3′) as described previously [xvi,17]. Sequence analysis of PCR products was conducted with MEGA version iii.one using the maximum likelihood method. AB695350 (S. maltophilia strain 4APB) was used as a command [18]. South. maltohpilia clinical isolates were typed using PFGE with Xba I digestion as described previously [19]. PFGE was performed with a CHEF-DR III apparatus (Bio-Rad Korea, Seoul, Korea) using 5 to 35 s of linear ramping at vi V/cm for xx h at 14°C. Digital images were analyzed with Fingerprinting Ii Informatix software (Bio-Rad, Hercules, CA, USA) using the Die coefficient and UPGMA with a 1% tolerance and 0.v% optimizing setting value. The results were interpreted using the criteria of Tenover et al. [twenty].
Antimicrobial susceptibilities and fractional inhibitory concentrations using a luciferase-based assay
An in vitro susceptibility test was performed for seven antimicrobial agents (ceftazidime, ciprofloxacin, levofloxacin, moxifloxacin, ticarcillin-clavulanic acid [TIM], tigecycline, and SXT) using the broth microdilution method co-ordinate to 2013 Clinical and Laboratory Standards Institute guidelines [21]. Quality controls were assessed by using Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. TIM was obtained from Biovim Korea Vine & Visitor (Seoul, Korea). Tigecycline was obtained from Pfizer Inc. (New York, NY, USA) via a chemical compound transfer program. Other antibiotics were obtained from Sigma-Aldrich (St. Louis, MO, United states of america). All susceptibility testing was performed using cation-adapted Mueller-Hinton broth (BD, Spark, MD, USA). To identify synergistic effects between SXT and other antibiotics (levofloxacin, moxifloxacin, or TIM), a checkerboard assay was performed using 96-well U-bottom microplates. Due to a previous report that broth microdilution endpoint for SXT are difficult to read because of trailing and bacteriostatic activity of S. maltophilia, a luciferase-based bacterial jail cell viability assay was used [22,23]. In this written report, a BacTiter-Glo™ microbial prison cell viability kit (Promega Corp., Madison, WI, USA) was used to make up one's mind the number of viable bacterial cells in civilisation, based on quantification of adenosine triphosphates (ATPs). Graded concentrations of antibiotics were mixed to appraise synergy test. Each well was inoculated with 5 × 104 CFU of each isolate in cation-adjusted Mueller-Hinton goop. The plates were then incubated for 24 h at 35°C in ambient air. All assays were performed in triplicate. After 24 h of incubation, a volume of BacTiter-Glo™ reagent equal to the volume of the jail cell culture medium was added to 100 μL of microbial broth civilization in an opaque-walled multi-well plate, according to the manufacturer's instructions. Relative luminescence units (RLU) were measured using a SpectraMax L luminescence microplate reader (Molecular Devices, Sunnyvale, CA, Us). The per centum of RLUs compared to the antibiotic-complimentary controls (%RLU) was calculated, with minimal inhibitory concentrations (MICs) divers equally <10%RLU, corresponding to an inhibitory concentration of 90% (IC90). Total fractional inhibitory concentrations (FIC) were calculated co-ordinate to the formula: ΣFIC = FIC of agent A + FIC of agent B, where FIC of agent A or B = MIC of agent A or B in combination/MIC of agent A or B alone. ΣFIC values ≤0.5 indicate synergy, ΣFIC values of >0.5 and ≤4 indicate indifference, and ΣFIC values >4 signal animosity [24].
Definitions
S. maltophilia BSIs were defined equally at to the lowest degree 1 S. maltophilia-positive blood civilisation in association with clinical signs or symptoms indicative of infection [six]. Polymicrobial BSIs were defined as the presence of an organism other than S. maltophilia in the same blood culture. The source of bacteremia was adamant clinically on the ground of the presence of an active site of infection every bit determined by chart review or isolation of the organism from other clinical specimens coincident with the episode of bacteremia [four]. Neutropenia was defined as an absolute neutrophil count (ANC) <500/mmiii, or <1000/mm3 with predicted falls to <500/mm3 within ii–iii days. Astringent neutropenia was defined as an ANC <100/mmiii [11,12]. The length of hospitalization before BSI was defined as the number of days from hospital access to the development of BSI. Previous antibiotic use was defined as the assistants of antibiotics for more than than 24 hours within 30 days before the onset of the S. maltophilia BSI [25]. Mortality was considered attributable to the S. maltophilia BSI in any of the following cases: (1) claret cultures positive for S. maltophilia at the time of death; (2) death before the resolution of signs and symptoms related to S. maltophilia BSI; (3) decease within 7 days of the onset of S. maltophilia BSI and with no other identifiable crusade [26]. Rough mortality was divers every bit mortality that occurred within a calendar month following a BSI episode [27].
Statistical assay
Differences in continuous variables betwixt the survivors and non-survivors were analyzed using the Mann–Whitney U-examination. Fisher'south exact examination was used to compare categorical data. Nosotros used Cox's proportional take a chance model with forward stepwise selection to identify contained risk factors for decease. Kaplan-Meier survival curves were used to clarify mortality trends. P value <0.05 was considered statistically meaning. All data were analyzed using SPSS ver. 18.0 (SPSS Korea, Seoul, Korea).
Results
Clinical characteristics of S. maltophilia bloodstream infection
Among 11,004 of admission episodes, a total of 31 patients were treated for South. maltophilia BSI. The incidence rate of South. maltophilia BSI was 0.134 cases per 1,000 patient-days during the entire study period. All patients had received wide-spectrum antibiotics such every bit fluoroquinolone as prophylaxis, anti-Pseudomonal cephalosporin plus aminoglycoside, or carbapenem equally empirical or targeted therapy due to neutropenic fever within xxx days before the onset of the BSIs. The most commonly identified source of BSI was pneumonia (41.ix%), followed past chief BSI (22.six%), catheter-related BSI (19.four%), skin and soft tissue infection (12.9%), and intra-abdominal infection (3.two%). In catheter-related Due south. maltophilia BSIs, Hickman catheters (north = 5) or chemoport (northward = 1) were removed from the patient. In addition, about one-third of the patients (35.5%) had polymicrobial BSIs with nosocomial pathogens such every bit methicillin-resistant Staphylococcus aureus, methicillin-resistant coagulase-negative Staphylococcus, P. aeruginosa or vancomycin-resistant Enterococcus. Although antibody regimens were modified to advisable targeted therapies in 26 of 31 patients (83.ix%), the overall and attributable mortality of S. maltophilia BSIs was 64.5% and 38.vii%, respectively.
Clinical characteristics of S. maltophilia BSIs were compared co-ordinate to overall survival condition (Table 1). There were no differences in age, sex, underlying hematologic diseases, SAPS II, presence of polymicrobial BSI, or shock betwixt the two groups. Neutropenia at the onset of BSI (43% vs. 92%, p =0.038) was significantly associated with death, in terms of both neutropenia itself and the elapsing of neutropenia (median 3 d vs. twoscore d, p =0.016). As a source of BSI, pneumonia (0% vs. 65.0%, p =0.001) was more common in not-survivors, while catheter-related infections (54.five% vs. 0%, p =0.001) were more mutual in survivors. In patients with combined S. maltophilia pneumonia, 64.5% (8 of 13 patients) received mechanical ventilation, while eleven.ane% (2 of xviii patients) with other sources of BSI received mechanical ventilation.
The results of Cox's proportional chance analysis of factors associated with overall bloodshed are shown in Table 2. Severe neutropenia at the onset of BSI (adjusted run a risk ratio [60 minutes] 5.24, 95% Conviction Interval [CI] 1.411-19.493; p =0.013) and daze (adjusted HR 3.15, 95% CI ane.231-8.032; p =0.017) at the onset of BSI, and pneumonia as a source of BSI (adjusted HR 6.05, 95% CI ii.247-16.291; p <0.001) were associated with an increase in mortality. Kaplan-Meier survival curves stratified by source of BSI were shown in Figure 1.
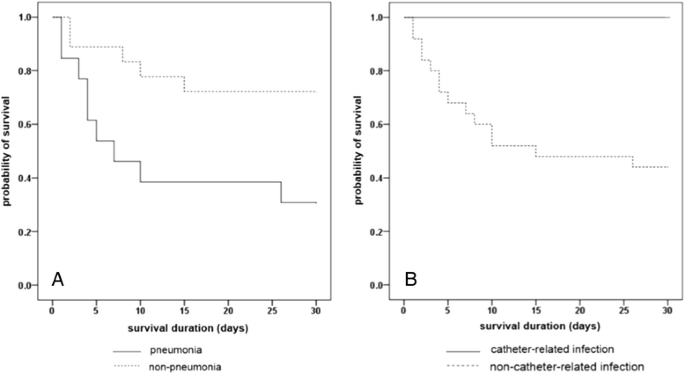
Kaplan-Meier curves for survival during episodes of Stenotrophomonas maltophilia bloodstream infections. Kaplan–Meier curves for survival stratified past pneumonia as a source of BSI (A), and catheter-related BSI (B) (Log-rank examination, p <0.001, and 0.003, respectively).
Pulsed-field gel electrophoresis and phylogenetic group determinations
PFGE typing of available 27Southward. maltophilia clinical isolates demonstrated 20 distinguishable banding patterns without evidence of the clonality. These 20 strains exhibited >99.9% 16S rRNA gene sequence similarity to the S. maltophilia type strain AB695350.
In vitro susceptibility testing and antimicrobial combinations
Broth microdilution testing of clinical isolates revealed 88.9% susceptibility to SXT and 44.4% to levofloxacin. MIC50 and MIC90 values for moxifloxacin and tigecycline showed ane and 4 mg/L, and 4 and 8 mg/L, respectively (Table three). Of the 27 clinical isolates, 15 were called for additional synergy testing based upon their MIC values. The selected isolates were representatives of the like MIC patterns to each antmicrobial agent. Comparison of FIC indices is shown in Table 4. Synergy betwixt SXT and TIM was found in 9 of xv strains (lx%) tested, of which the FIC for fifty and 90% of the isolates (FIC50/FICxc) was 0.500/0.750, with a range of 0.254 to 1.500. In the instance of SXT plus levofloxacin or moxifloxacin, FICs ranged from 0.500 to 1.000, and 0.313 to 0.750, respectively. In all of the antimicrobial combinations tested, based on the SXT, the FIC90 values were under i.000 without animosity.
Discussion
In this study, we examined the clinical characteristics and treatment outcomes in hematologic patients with S. maltophilia BSIs, as well as the effectiveness of in vitro antimicrobial combinations confronting Southward. maltophilia clinical isolates. S. maltophilia BSIs withal showed high mortality, with meaning correlations seen for severe neutropenia, shock, and concomitant Due south. maltophilia pneumonia. Furthermore, we discovered that in vitro synergy tests revealed favorable FICfifty and FIC90 values against S. maltophilia clinical isolates obtained from hematologic patients.
Our data demonstrated that the previous exposure to broad spectrum antibiotics was preceded in all of the S. maltophilia BSIs. Polymicrobial infections were observed in over one third of patient. Poor prognosis in patients with combined Due south. maltophilia pneumonia might exist related to higher rates of mechanical ventilation, when compared to patients with other sources of BSI. In contrast, patients with catheter related BSIs exhibited 100% survival in this study. Favorable outcomes in these cases are likely to be associated with the removal of the catheter. A previous retrospective study found that catheter-related South. maltophilia BSIs were cured later removal of the catheter, even without appropriate antibiotics therapy, while a different written report observed a significant correlation between bloodshed and retained catheters [25,28]. However, these studies were limited past the small number of patients examined, and failed to evaluate other salvage direction such equally antibiotics lock therapy in patients using long-term catheters.
In a worldwide report of the antimicrobial susceptibilities of 1,586 clinical Southward. maltophilia isolates, SXT and tigecycline showed susceptibilities of 96.0% and 95.5%, respectively, followed by levofloxacin with a susceptibility of 83.4% [29]. A recent written report reported no difference in 30-twenty-four hours mortality between SXT and levofloxacin treatment for South. maltophilia BSIs [10]. However, in vitro susceptibility results from our study besides revealed characteristics indicative of patient therapies. Decreased susceptibility to levofloxacin was associated with prophylactic fluoroquinolone use during the chemotherapy or SCT for hematologic malignancies. Taken together, levofloxacin may exist an appropriate alternative handling for S. maltophilia infections though conscientious patient selection based on the history of previous fluoroquinolone exposure volition exist necessary. For SXT, the susceptibility charge per unit was 88.9% using the broth microdilution method, compared to 93.9% using an automated system (Vitek-2, bioMérieux, Hazelwood, MO, USA). This difference is probable the consequence of the inoculum issue and abaft. The MIC50/90 for moxifloxacin and tigecycline also showed higher values, and thus needs farther evaluation for the clinical use in this grouping of patients.
In vitro synergy was screened for SXT in combination with other antimicrobial agents. Near ninety% of South. maltophilia isolates were susceptible to SXT, though several reports of emerging resistance to SXT have been found [29]. We included TIM as a representative of the beta-lactam antibiotics, as a upshot of our in vitro susceptibility tests. Fluoroquinolone was selected for synergy examination due to its widespread clinical employ. Another reason to choose quinolone as a combination antibiotics was the possible activity against biofilm formation in device-related infection or in cystic fibrosis patients [30,31]. Further studies are needed to identify the biofilm activity of quinolone in hematologic patients. Our study demonstrated that SXT plus TIM exhibited the highest rates of synergy amidst the antibiotic combinations tested. Both SXT plus levofloxacin and SXT plus moxifloxacin revealed FIC beneath 1.000 against all of the clinical isolates tested. Combination therapy may, therefore, correspond a viable option for S. maltophilia BSIs. Further studies with larger number of patients will be needed to assess whether the combination therapy has clinical impact in improving outcomes of S. maltophilia BSI in hematologic patients.
There are several strengths of this written report. Commencement, just BSI cases were included. In non-bacteremic S. maltophilia infection, it is difficult to distinguish the colonization from infection, which might influence the outcome analysis. 2nd, we calculated the incidence rate of S. maltophilia BSI in hematologic patients. 3rd, we used a high-throughput bioluminescence assay to assess the viability in living organisms, and a luciferase-based assay for determining the number of feasible cells in culture [32]. The application of the luciferase-based assay for the measurement of FIC indices was used to overcome difficulties in reading due to trailing endpoint by converting MIC to %RLU, equivalent to ICxc values.
Conclusion
In conclusion, S. maltophilia BSIs shows high mortality in patients with hematologic malignancies. Neutropenia, shock, and combined S. maltophilia pneumonia are associated with mortality. Based upon drug susceptibility testing, the primary treatment of pick in hematologic patients should be SXT, with combination therapies including SXT serving as a feasible handling option for Due south. maltophilia BSIs.
References
-
Safdar A, Rolston KV. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis. 2007;45:1602–9.
-
Looney WJ, Narita M, Mühlemann Yard. Stenotrophomonas maltophilia: an emerging opportunist homo pathogen. Lancet Infect Dis. 2009;ix:312–23.
-
Kwon JC, Kim SH, Choi JK, Cho SY, Park YJ, Park SH, et al. Epidemiology and clinical features of bloodstream infections in hematology wards: one year experience at the catholic claret and marrow transplantation heart. Infect Chemother. 2013;45:51–61.
-
Araoka H, Baba M, Yoneyama A. Risk factors for bloodshed among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Nihon, 1996–2009. Eur J Clin Microbiol Infect Dis. 2010;29:605–8.
-
Senol E, DesJardin J, Stark PC, Barefoot Fifty, Snydman DR. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis. 2002;34:1653–vi.
-
Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia: a seven-yr review. J Hosp Infect. 2012;81:114–8.
-
Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, et al. Targeted therapy confronting multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011). Haematologica. 2013;98:1836–47.
-
Falagas ME, Valkimadi PE, Huang YT, Matthaiou DK, Hsueh PR. Therapeutic options for Stenotrophomonas maltophilia infections beyond co-trimoxazole: a systematic review. J Antimicrob Chemother. 2008;62:889–94.
-
Lee CS, Doi Y. Therapy of infections due to carbapenem-resistant gram-negatice pathogens. Infect Chemother. 2014;46:149–64.
-
Cho SY, Kang CI, Kim J, Ha YE, Chung DR, Lee NY, et al. Can levofloxacin be a useful culling to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother. 2014;58:581–three.
-
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the apply of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–93.
-
Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Prove-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011;26:220–52.
-
Ito JI, Tegtmeier BR, O'Donnell MR. Antibacterial prophylaxis in patients with cancer and neutropenia. N Engl J Med. 2006;354:ninety–4.
-
Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25:ii–41.
-
Church D, Lloyd T, Peirano G, Pitout J. Antimicrobial susceptibility and combination testing of invasive Stenotrophomonas maltophilia isolates. Scand J Infect Dis. 2013;45:265–70.
-
Whitby PW, Carter KB, Burns JL, Royall JA, LiPuma JJ, Stull TL. Identification and detection of Stenotrophomonas maltophilia by rRNA-directed PCR. J Clin Microbiol. 2000;38:4305–9.
-
Adamek Chiliad, Overhage J, Breast-stroke S, Wintertime J, Fischer R, Schwartz T. Genotyping of ecology and clinical Stenotrophomonas maltophilia isolates and their pathogenic potential. PLoS Ane. 2011;half-dozen:e27615.
-
Kumar S, Tamura K, Nei M. Mega3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–63.
-
Denton Grand, Todd NJ, Kerr KG, Hawkey PM, Littlewood JM. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–8.
-
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray Exist, Persing DH, et al. Interpreting chromosomal DNA brake patterns produced past pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9.
-
Clinical and Laboratory Standards Institute. Operation standards for antimicrobial susceptibility testing; twenty-second advisory supplement. CLSI certificate M100-S23. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2013.
-
Poulos CD, Matsumura So, Willey BM, Low DE, McGeer A. In vitro activities of antimicrobial combinations against Stenotrophomonas (Xanthomonas) maltophilia. Antimicrob Agents Chemother. 1995;39:2220–iii.
-
Krueger TS, Clark EA, Nix DE. In vitro susceptibility of Stenotrophomonas maltophilia to various antimicrobial combinations. Diagn Microbiol Infect Dis. 2001;41:71–eight.
-
Moody JA. Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In: Garcia LS, Isenberg HD, editors. Clinical microbiology procedures handbook. Washington, DC: ASM Press; 2007. p. 1–23.
-
Lai CH, Wong WW, Chin C, Huang CK, Lin HH, Chen WF, et al. Central venous catheter-related Stenotrophomonas maltophilia bacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clin Microbiol Infect. 2006;12:986–91.
-
Kim SH, Yoon YK, Kim MJ, Shon JW. Risk factors for and clinical implications of mixed candida/bacterial bloodstream infections. Clin Microbiol Infect. 2013;19:62–8.
-
Ortega M, Rovira Chiliad, Almela 1000, Marco F, de la Bellacasa JP, Martínez JA, et al. Bacterial and fungal bloodstream isolates from 796 hematopoietic stem prison cell transplant recipients between 1991 and 2000. Ann Hematol. 2005;84:xl–6.
-
Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect. 2002;45:47–53.
-
Farrell DJ, Sader HS, Jones RN. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrob Agents Chemother. 2010;54:2735–seven.
-
Di Bonaventura K, Spedicato I, D'Antonio D, Robuffo I, Piccolomini R. Biofilm germination past Stenotrophomonas maltophilia: modulation past quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob Agents Chemother. 2004;48:151–lx.
-
Wu K, Yau YC, Matukas L, Waters V. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2013;57:1546–eight.
-
Fan F, Wood KV. Bioluminescent assays for loftier-throughput screening. Assay Drug Dev Technol. 2007;five:127–36.
Acknowledgements
Role of this study was presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy in Denver, CO, The states, September 10–thirteen, 2013 (Poster No. E612). We would like to give thanks Biovim Korea Vine & Company (Seoul, Korea) for providing the ticarcillin-clavulanic acid used in this written report. We likewise similar to thank Pfizer Inc. (New York, NY, USA) for providing tigecycline via a compound transfer programme.
Author information
Affiliations
Respective author
Additional information
Competing interests
This study was supported past a grant from the Korean Health Applied science R&D Projection, Ministry of Health & Welfare, South korea (Grant No. HI12C0756). The authors declare that they have no competing interests.
Authors' contributions
SYC conducted the infirmary chart review and analysis of resulting data, interpreted data, drafted the initial study study and wrote the final written report. DGL conceptualized the study and contributed to information interpretation. He revised and edited the manuscript. SMC and SHP participated in critical revision of manuscript. CP and HSC performed laboratory work and contributed to interpret the results. JKC, HJL, JHC and JHY contributed the data estimation and editing the manuscript. YJ Park contributed the interpretation of microbiologic data. All authors read and approved the last manuscript.
Rights and permissions
Open Access This article is licensed nether a Artistic Eatables Attribution 4.0 International License, which permits utilize, sharing, adaptation, distribution and reproduction in whatever medium or format, as long every bit you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the commodity'due south Creative Commons licence, unless indicated otherwise in a credit line to the textile. If material is non included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission directly from the copyright holder.
To view a re-create of this licence, visit https://creativecommons.org/licenses/by/4.0/.
The Artistic Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/aught/one.0/) applies to the data fabricated bachelor in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
About this commodity
Cite this article
Cho, SY., Lee, DG., Choi, SM. et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 15, 69 (2015). https://doi.org/10.1186/s12879-015-0801-7
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/x.1186/s12879-015-0801-7
Keywords
- Bacteremia
- Drug combinations
- Hematologic diseases
- Bloodshed
- Stenotrophomonas maltophilia
Source: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-015-0801-7
0 Response to "Bloodstream Infections Caused by Stenotrophomonas Maltophilia a Seven-year Review"
Postar um comentário